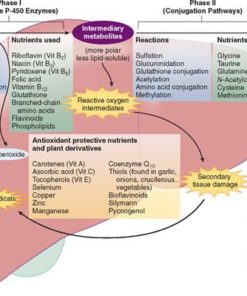
Homocysteine - Understanding Homocysteine and Methylation: A Functional Medicine Perspective
Homocysteine sits at the center of methylation pathway, pivoting the body's biochemistry in one direction or another - towards detoxification, and neurotransmitter regulation or inflammation. In functional and Walsh-based biochemical medicine, homocysteine is not simply a cardiovascular marker, it is a key amino acid reflecting how efficiently the body produces SAMe, clears oxidative stress, builds glutathione, and regulates neurologic stability.
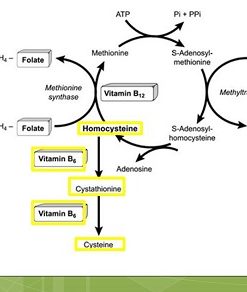
Homocysteine is formed when S-adenosylhomocysteine (SAH) is broken down. What happens next depends on the person’s nutrional status and determines one's methylation performance:
-
Undermethylators with elevated homocysteine, tend to accumulate SAH. It also reflects a block in coverting either to glutathione or to methionine, or both.
-
Overmethylators tend to push homocysteine rapidly back into methionine or cystathionine, sometimes causing low levels of homocysteine.
Whether homocysteine moves upward toward methionine and SAMe, or downward toward cysteine and glutathione, is determined by nutrient cofactors—especially vitamin B6, B12, folate, zinc, and TMG.
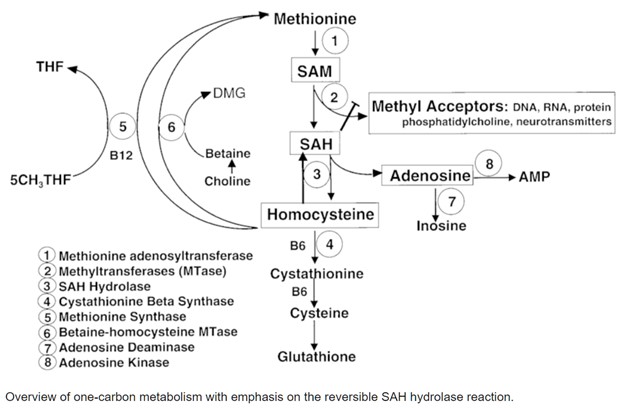
How Homocysteine Is Formed: The SAH → Homocysteine Connection
S-adenosylhomocysteine (SAH) is produced when SAMe donates a methyl group to creatine formation, DNA, neurotransmitters, or hormones. SAH then breaks down into homocysteine. This step is crucial because:
-
Elevated SAH is more toxic than homocysteine itself.
-
SAH is a direct inhibitor of methylation reactions.
-
Clearing SAH requires converting it to homocysteine and then moving homocysteine in the correct direction; or to homocysteine disposal by the kidneys.
This means that high homocysteine often reflects deeper methylation congestion, not just a nutritional problem.
Homocysteine, Methionine, and Glutathione: Two Pathways, Two Outcomes
Once homocysteine is produced, it has two routes:
1. Remethylation → Back to Methionine (Requires B12 and/or Folate and/or TMG)
This pathway supports:
-
Methionine → SAMe production
-
DNA methylation
-
Neurotransmitter balance
-
Stress response
Undermethylators often struggle here due to high SAH or low cofactors. Zinc is a cofactor necessary in the enzymatic (SAHH) conversion of SAH to homocysteine.
2. Transsulfuration → Toward Cysteine and Glutathione (Requires Vitamin B6 and P5P)
This pathway supports:
-
Glutathione synthesis
-
Detoxification
-
Anti-inflammatory defenses
-
Mitochondrial protection
Vitamin B6/ P5P is essential for lowering homocysteine by pushing it into glutathione production.
In undermethylators—common in OCD, depression, inner tension, high histamine states—homocysteine often becomes trapped, with neither pathway working efficiently.
Homocysteine in Undermethylators: Why It Builds Up
Undermethylators tend to show:
-
Elevated SAH
-
Elevated or “stagnant” homocysteine
-
Low methionine (poor conversion via enzymes regulated by B12, folates or TMG)
-
Low SAMe (low cellular energy - mitochondrial function to convert methionine to SAM)
-
Reduced glutathione production (low B6, P5P or cysteine)
-
High oxidative stress and inflammation
Because SAH inhibits methylation, the body struggles to recycle homocysteine back into methionine. Without adequate B6, P5P, cysteine and zinc, converting downward into glutathione is also impaired.
This is why in undermethylators, supporting:
- Methionine
- SAMe production
- Creatine
- Alkalinity
- Vitamin B6
- Zinc
- Antioxidants
creates predictable improvements in both homocysteine levels and mood regulation.
How to Lower Homocysteine with Vitamin B6, Methyl Donors, and Targeted Nutrients
Lowering homocysteine is not simply about adding more folate or B vitamins. The direction it needs to move depends on the individual’s methylation profile.
Nutrients that Lower Homocysteine (via Transsulfuration)
-
Vitamin B6 (P5P):
Required for converting homocysteine → cystathionine → cysteine → glutathione.
Most clinically effective for lowering homocysteine in undermethylators. -
N-acetylcysteine (NAC):
Supports glutathione synthesis and lowers oxidative stress. -
Glycine and serine:
Support the transsulfuration branch and glutathione pathway.
Nutrients that Recycle Homocysteine to Methionine (via Methylation)
-
B12 (methylcobalamin or hydroxocobalamin)
-
Folate (methyl or folinic, depending on tolerance)
-
TMG (trimethylglycine / betaine):
Donates a methyl group directly to homocysteine. -
Zinc:
A required cofactor for SAH → homocysteine processing and for lowering SAH.
Additional Nutrients that Support Both Pathways
-
Magnesium:
Required for dozens of methylation and ATP-dependent reactions. -
Antioxidants (C, E, selenium):
Protect against oxidative stress that elevates homocysteine.
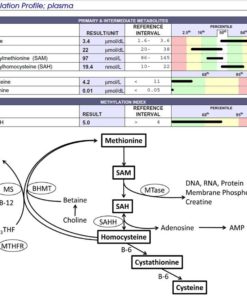
Lab Tests That Reveal How Homocysteine Interacts With Methylation and Inflammation
To understand how homocysteine is behaving, the most informative tests are:
-
Homocysteine (plasma)
-
SAM and SAH levels (Doctors Data Panel)
-
Methionine
-
B6, B12, folate
-
Glutathione markers
- CBC - identifies if macrocytosis is present
-
MMA (for functional B12 status)
-
Histamine (as a methylation phenotype marker)
-
Genetic markers (MTHFR, MTR, MTRR, CBS)
For undermethylators, SAH and methionine are typically more revealing than homocysteine alone.
Diet, Lifestyle, and Functional Medicine Strategies to Support Homocysteine Balance
-
Emphasize protein, eggs, zinc-rich foods, and sulfur-rich vegetables.
-
Reduce refined carbohydrates and processed foods, which drive oxidative stress.
-
Support gut integrity: dysbiosis increases oxidative load and SAH accumulation.
-
Use intermittent fasting or ketogenic strategies when appropriate—both reduce inflammation and improve methylation efficiency.
-
Support sleep and circadian rhythm to lower inflammation.
Conclusion: Homocysteine and Methylation Are Two Sides of the Same Metabolic Coin
Homocysteine is not merely a cardiovascular marker—it is a central indicator of methylation efficiency, SAH accumulation, methionine recycling, glutathione production, and overall inflammatory load.
Understanding homocysteine through the lens of methylation explains why some individuals—especially undermethylators—struggle with mood disorders, oxidative stress, and chronic inflammation even when diet seems adequate.
By supporting the correct pathways with vitamin B6, zinc, B12, folate (when not contraindicated), methionine, TMG, and glutathione precursors, homocysteine can be directed into the pathway that best serves the individual’s biochemical pattern.