Evaluating Methylation Status: MTHFR vs Methylation Pathway Testing in Depression
Testing for Depression": Whole Blood Histamine in the Walsh Approach and Its Role as an Initial Assessment for Methylation Status
In the Walsh Approach, "testing for depression" sometimes involves whole blood histamine as the initial test to assess an individual's methylation status. Elevated levels of histamine in the blood are generally indicative of undermethylation, while low levels suggest overmethylation. However, this test can sometimes produce ambiguous results when patients are concurrently taking antihistamines or certain antidepressants. In such cases, the Doctors Data (DD) Methylation Panel can provide a more reliable assessment. Although it requires special blood drawing and handling procedures and is not typically recommended for overmethylators, the DDI panel serves as a valuable tool in determining methylation status for undermethylators and normomethylators.
The DD Methylation Panel: "Evaluating Markers in the Methylation Pathway
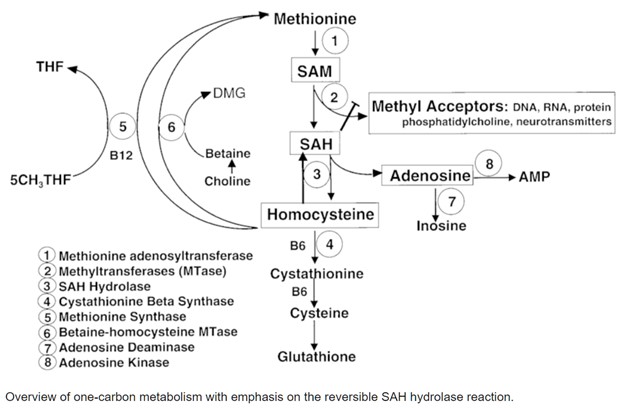
The DD Methylation Panel, by "evalating plasma methylation markers", delves deeper than a conventional MTHFR genetic test by measuring actual levels of key metabolites involved in the methylation cycle, including S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), methionine, and homocysteine. Of particular interest is the ratio of SAM to SAH, which provides a clear indicator of methylation capacity. SAM serves as the primary methyl group donor in the body, while SAH, its product after donation, inhibits SAM activity. An unfavorable SAM:SAH ratio can thus hint at an inhibition of methylation activity.
Additionally, homocysteine levels offer insight into both transsulfuration pathways leading to glutathione synthesis and potential impairments in its conversion to methionine, possibly due to deficiencies in B12 or folate. By examining these key markers, the DDI panel helps identify potential genetic SNPs contributing to methylation impairment, providing a more comprehensive view of an individual's methylation status.
Methylation Pathway: Unraveling Its Role in "Cause of Mood Disorders" and Other Health Conditions
The methylation pathway, also known as the one-carbon cycle, plays a vital role in numerous biological functions. This includes DNA repair, gene expression, detoxification processes, immune system regulation, and the synthesis and breakdown of neurotransmitters. Disruptions in this pathway, resulting in poor methylation, can contribute significantly to various conditions such as depression, OCD, autism, oppositional defiance, and extreme religiosity. These conditions, often considered a "cause of mood disorders", are commonly associated with imbalances in serotonin and dopamine reuptake, hence the importance of assessing an individual's methylation status.
MTHFR vs One Carbon Cycle Testing": Utilizing Methylation Pathway Insights for Personalized Treatment
Understanding an individual's methylation pathway, sometimes framed as "MTHFR vs One Carbon Cycle testing", can offer invaluable insights for personalized treatment. For instance, identifying an undermethylation status can guide the implementation of tailored nutrient support aimed at optimizing methylation processes, which can help alleviate associated symptoms. This approach presents a promising avenue in managing conditions often linked to methylation imbalances, enhancing the body's natural capacity for mood regulation, cognitive function, and overall mental well-being.
Evaluating DD Methylation Panel in Depression for Better Outcomes
The DD Methylation Panel shines in its capacity to offer dynamic, real-time insights into an individual's methylation activity by "evaluating plasma methylation markers". Unlike a static genetic test that merely highlights potential genetic vulnerabilities, the DDI panel considers the functional outcomes of these genetic factors along with nutritional, environmental, and lifestyle influences. It provides a practical, actionable understanding of an individual's methylation status, enabling healthcare providers to devise more effective, personalized treatment strategies. These may involve targeted nutrient support to rectify any imbalances, ultimately leading to improved health outcomes for individuals suffering from conditions attributed to methylation imbalances.
