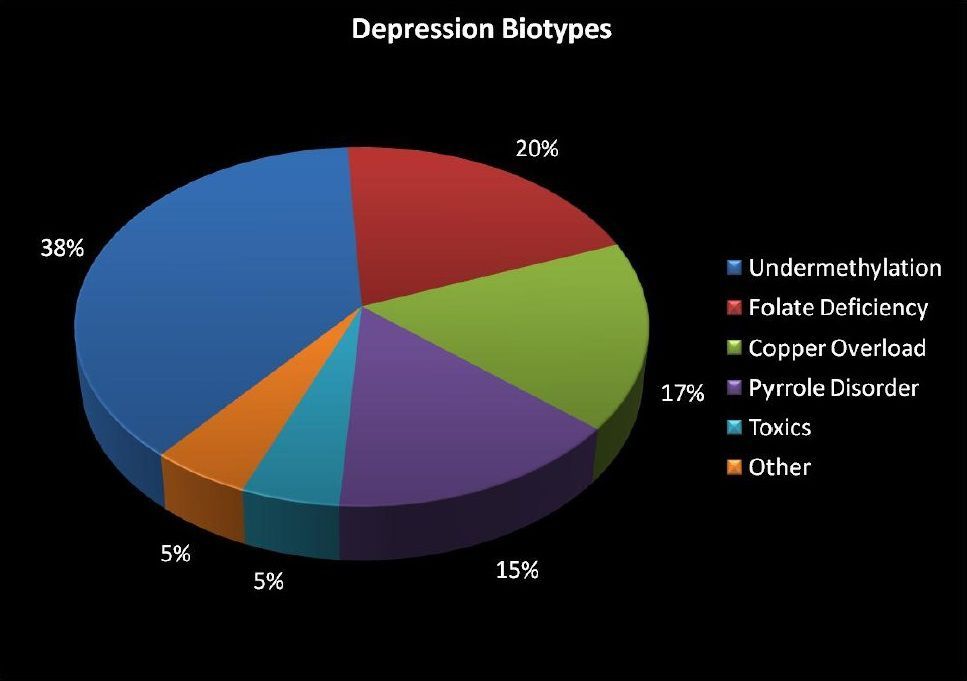
Five Biotypes of Depression — Nutrition and Depression
The concept of the Five Biotypes of Depression emerged from decades of clinical and laboratory research in biochemical psychiatry focused on understanding why depression does not behave as a single condition. Rather than beginning with psychiatric diagnoses, Dr. William J. Walsh, PhD. developed this framework by analyzing patterns of nutrient imbalance, methylation status, trace metal regulation, and oxidative stress in large populations of patients with depression and anxiety.
Walsh’s work drew on earlier orthomolecular psychiatry and trace-element research, integrating laboratory indicators—such as whole-blood histamine, zinc and copper levels, ceruloplasmin, and homocysteine—with consistent emotional, behavioral, and treatment-response patterns. Over time, these recurring biochemical signatures revealed that depression clusters into a limited number of reproducible biochemical profiles, now described as the Five Biotypes of Depression, each defined by a distinct mechanism affecting neurotransmitter regulation rather than by symptom severity alone.
A core principle of the Walsh Protocol is that nutrition and depression are inseparably linked through biochemistry, not through generalized dietary advice. Neurotransmitter synthesis, transport, receptor sensitivity, and clearance are all regulated by nutrient-dependent enzymes and methylation processes. As a result, effective treatment depends on individualized nutrient management, guided by laboratory markers and symptom patterns that identify the dominant biochemical biotype.
This framework explains why patients with the same diagnosis may respond in opposite ways to the same medication or supplement. Nutrients that stabilize neurotransmission in one biotype may worsen anxiety or depression in another. The Walsh Protocol therefore emphasizes precise selection, avoidance, and titration of nutrients—such as folates, zinc, vitamin B6, niacinamide, and related compounds—based on the patient’s underlying biochemical pattern. Secondary influences, including inflammation, intestinal health, toxic exposure, hormone signaling, and mitochondrial function, are addressed only in ways that support this foundational biochemical model rather than override it.
UNDERMETHYLATION — Nutrition and Depression
In the Walsh Protocol, undermethylation represents a biochemical pattern defined by insufficient effective methylation capacity, most accurately reflected by a reduced SAM:SAH ratio. While SAM (S-adenosylmethionine) provides methyl groups for critical biochemical reactions, relative elevation of SAH suppresses methyltransferase activity, limiting the brain’s ability to regulate neurotransmitters, gene expression, and systemic detoxification processes. Depression in this biotype arises from chronically low functional monoamine activity, particularly serotonin and dopamine.
A hallmark laboratory feature of undermethylation is elevated whole-blood histamine. Walsh used histamine as a functional marker because histamine is normally inactivated through methylation. When methylation capacity is insufficient, histamine clearance is impaired, leading to elevated levels. High histamine is therefore not causal in itself, but a reliable indicator of inadequate methylation efficiency, correlating with characteristic symptoms such as inner tension, obsessive or rigid thinking, seasonal depression, rumination, and anger under stress.
At the neurochemical level, Walsh emphasized that undermethylation involves reduced synaptic availability of serotonin and dopamine, not excessive neurotransmitter activity. A critical and often misunderstood mechanism involves epigenetic regulation of neurotransmitter transporters. Methylation influences histone modification and gene expression governing monoamine transporters such as SERT (serotonin transporter) and DAT (dopamine transporter). In undermethylated individuals, baseline monoamine signaling is already low; interventions that increase transporter expression or activity can further accelerate neurotransmitter reuptake and clearance.
This explains a central Walsh observation: folates frequently worsen symptoms in undermethylation, not because they “overcorrect” methylation, but because folates can promote histone-mediated upregulation of monoamine transporter expression, increasing serotonin and dopamine clearance from the synapse. The result is further reduction in functional monoamine signaling and worsening of depressive or obsessive symptoms. This transporter-centric explanation is foundational to the Walsh model.
Beyond neurotransmitter regulation, undermethylation has systemic implications. Methylation is required for:
• DNA and histone methylation
• Neurotransmitter synthesis and metabolism
• Histamine clearance
• Phospholipid and myelin maintenance
• Detoxification reactions
• Endogenous creatine synthesis
In many undermethylated individuals, creatine demand is elevated, making creatine synthesis one of the largest consumers of available methyl groups. When methionine intake is normal but SAM remains low and symptoms of high creatine demand are present, ongoing creatine synthesis can further depress methyl availability and exacerbate low SAM:SAH ratios. This contributes to fatigue, low motivation, and poor stress tolerance frequently seen in this biotype.
Walsh treatment emphasizes individualized nutrient management to restore functional monoamine activity without increasing transporter-mediated clearance. This typically includes zinc, vitamin B6, and niacinamide, while avoiding folates and methyl donors that worsen neurotransmitter availability. The goal is not to maximize methylation indiscriminately, but to optimize methylation efficiency and distribution so that neurotransmitter regulation, epigenetic control, detoxification, and energy metabolism can function in balance.
In this context, nutrition and depression are inseparable from biochemical precision. Effective treatment depends on recognizing undermethylation as a disorder of methylation efficiency and neurotransmitter regulation, guided by the SAM:SAH ratio, histamine status, and characteristic symptom patterns, rather than by diagnosis alone.
OVERMETHYLATION — Nutrients for Depression and Anxiety
In the Walsh Protocol, overmethylation represents a biochemical pattern characterized by relatively high methylation activity, most often reflected by low whole-blood histamine and a high or efficient SAM:SAH ratio. In this biotype, methylation reactions proceed readily, and symptoms arise not from deficient neurotransmitter synthesis, but from excess or poorly regulated monoamine signaling, particularly within serotonergic pathways.
Walsh used low histamine as a functional marker because histamine is normally degraded through methylation. When methylation capacity is high, histamine clearance is efficient, resulting in low circulating levels. Clinically, this correlates with a pattern dominated by anxiety, panic, inner agitation, sensory and chemical sensitivities, insomnia, and emotional reactivity, often accompanied by depressive symptoms secondary to chronic overstimulation.
At the neurochemical level, overmethylation differs fundamentally from undermethylation in its relationship to neurotransmitter transporters. Methylation influences histone modification and gene expression governing monoamine transporters such as SERT (serotonin transporter) and DAT (dopamine transporter). In overmethylated individuals, baseline serotonergic activity is often excessive or poorly buffered. Increasing transporter expression or activity can therefore be therapeutically beneficial, as it accelerates serotonin reuptake and reduces synaptic overstimulation.
This mechanism explains a core Walsh principle: folates are often helpful in overmethylation, in direct contrast to their effect in undermethylation. Through histone-mediated effects on transporter expression, folates can increase SERT activity, lowering synaptic serotonin levels and thereby reducing anxiety, panic, and sensory overload. This biotype-specific response underscores why folate tolerance is a diagnostic clue rather than a universal recommendation.
Niacin and niacinamide play a distinct and complementary role in this pattern. Unlike folates, niacin does not act primarily through transporter regulation. Instead, it functions as a methyl-consuming nutrient, increasing demand on SAM and thereby moderating excessive methylation pressure. Walsh used niacin strategically to calm overmethylated states, particularly when anxiety and agitation predominate, while avoiding indiscriminate use that could destabilize other biotypes.
Beyond neurotransmission, elevated methylation capacity in overmethylation also affects systemic processes, including DNA and histone methylation, histamine metabolism, and detoxification reactions. When methylation is excessive or unevenly distributed, symptoms may extend beyond mood to include chemical sensitivities, adverse medication reactions, and exaggerated responses to dietary or environmental stimuli. These features reflect heightened neuroimmune and sensory reactivity, rather than simple neurotransmitter imbalance.
Within the Walsh framework, treatment focuses on reducing excessive monoamine signaling and moderating methylation activity, while avoiding interventions designed for undermethylation. Nutrient strategies are selected based on histamine status, symptom pattern, and response, with careful attention to folate tolerance, niacin effects, and zinc balance. The objective is not to suppress methylation globally, but to restore balanced regulation of neurotransmitters and gene expression so that both depression and anxiety can resolve without provoking further instability.
COPPER OVERLOAD — Nutrients for Anxiety
In the Walsh Protocol, copper overload is defined by excess free (unbound) copper relative to zinc and ceruloplasmin, rather than by total copper alone. It is the free copper fraction that disrupts neurotransmitter regulation and produces a pattern of anxiety-dominant depression, emotional volatility, and poor stress tolerance.
Copper exerts its neuropsychiatric effects primarily by stimulating dopamine β-hydroxylase, the enzyme responsible for converting dopamine into norepinephrine. Elevated free copper therefore drives excess norepinephrine activity, locking the nervous system into a persistent fight-or-flight state. At the same time, copper interferes with inhibitory neurotransmission, reducing effective GABA modulation. This combination—heightened excitatory signaling with diminished inhibition—explains why copper overload reliably presents with anxiety, panic, agitation, and insomnia rather than isolated low-mood states.
Walsh emphasized that copper toxicity is closely tied to ceruloplasmin function and metallothionein activity. Ceruloplasmin binds and transports copper safely; when ceruloplasmin is low or dysfunctional, copper remains biologically active. Metallothioneins—zinc-dependent metal-binding proteins—play a critical role in sequestering copper and limiting its reactivity. Impaired metallothionein expression allows copper to accumulate and circulate in its free form.
Zinc is therefore central to treatment, as it is required for both metallothionein synthesis and regulation of copper absorption. Selenium also contributes indirectly by supporting antioxidant defenses that limit copper-induced oxidative stress. Walsh’s laboratory focus includes assessment of copper, ceruloplasmin, and zinc status to estimate free copper burden and guide individualized nutrient correction.
Several common conditions predispose to copper overload, including estrogen dominance, use of oral contraceptives, copper-containing IUDs, pregnancy and postpartum hormonal shifts, obesity, environmental exposure such as well water, and genetic differences affecting copper transport or metallothionein expression. Walsh also noted that total heavy-metal burden can impair copper removal by competing for metallothionein binding, contributing to persistent symptoms or sluggish response to supplementation.
Treatment within the Walsh Protocol centers on zinc-based normalization of copper handling, with use of metallothionein-promoting strategies in resistant cases rather than aggressive chelation. Second Opinion Physician builds on this framework by incorporating inositol hexaphosphate (IHP) as a targeted gut-level mineral-binding adjunct, helping reduce ongoing copper absorption and total metal burden while supporting the core Walsh strategy of restoring physiological copper regulation.
PYRROLE DISORDER — Nutrient Deficiency and Mental Health
Pyrrole disorder (pyroluria or kryptopyrroluria) is a biochemical vulnerability with profound downstream effects, not because pyrroles themselves are toxic, but because of their predictable and sustained disruption of zinc, copper, and vitamin B6 homeostasis. Historically recognized in psychiatry—particularly in schizophrenia and severe mood disorders—pyrrole disorder represents a stress-driven disturbance in heme metabolism that creates ongoing nutrient loss and secondary neurochemical instability.
Pyrroles are byproducts of heme synthesis, generated during hemoglobin turnover and other heme-dependent processes. Under conditions of genetic susceptibility, oxidative stress, inflammation, or psychological trauma, excess pyrrole compounds are produced. These pyrroles bind zinc and vitamin B6 with high affinity, promoting their urinary excretion and creating a chronic functional deficiency independent of dietary intake.
The most clinically significant consequence of this process is progressive disruption of zinc-dependent regulation of copper. Zinc is required for metallothionein synthesis and for proper control of copper absorption, transport, and sequestration. As zinc is depleted, copper regulation deteriorates, often resulting in elevated free copper and reduced ceruloplasmin activity, even when total copper levels appear only mildly abnormal. For this reason, pyrrole disorder frequently drives or exacerbates copper overload, amplifying anxiety, emotional volatility, and stress intolerance.
From a Walsh perspective, identifying pyrrole disorder without assessing zinc, copper, and ceruloplasmin is incomplete and potentially misleading. The severity of symptoms correlates less with pyrrole levels alone than with the degree of zinc depletion, copper dysregulation, and ceruloplasmin impairment that has developed over time. Any patient suspected of pyrrole disorder should therefore understand their copper-to-zinc balance and ceruloplasmin status prior to treatment, as these markers determine both symptom expression and treatment strategy.
Clinically, pyrrole disorder is associated with severe stress intolerance, inner tension, social anxiety or withdrawal, irritability under pressure, emotional lability, and episodic decompensation, often triggered by illness, inflammation, or psychological stress. Physical features may include white spots on fingernails, poor wound healing, digestive sensitivity, frequent infections, fatigue, and musculoskeletal discomfort, reflecting the disorder’s systemic reach.
Inflammation further compounds this pattern. Zinc and B6 depletion impair immune regulation and intestinal barrier integrity, increasing inflammatory signaling and oxidative stress. Inflammation, in turn, increases pyrrole production, creating a self-reinforcing cycle that accelerates zinc loss and copper dysregulation. This cycle explains why pyrrole disorder often worsens during periods of stress, infection, or environmental exposure.
Walsh also emphasized the importance of essential fatty acid balance in pyrrole disorder, particularly the role of arachidonic acid (AA). Chronic zinc and B6 deficiency disrupt fatty acid metabolism, frequently leading to low omega-6 status, including inadequate AA. Because AA is required for neuronal membrane integrity, neurotransmitter receptor signaling, and controlled inflammatory resolution, deficiency can worsen both mood instability and inflammatory persistence. This underscores Walsh’s principle of biochemical individuality, including in fatty acid requirements.
Within the Walsh Protocol, treatment of pyrrole disorder centers on carefully titrated zinc and vitamin B6 repletion, guided by symptom response and mineral balance rather than fixed dosing. However, Walsh stressed that copper status must be monitored and addressed concurrently, as correcting zinc deficiency often unmasks or mobilizes copper. The clinical priority is restoring zinc-dependent regulation of copper and ceruloplasmin, not merely suppressing pyrrole excretion.
In this context, nutrient deficiency and mental health are inseparable from mineral balance. Pyrrole disorder explains why some individuals exhibit extraordinary sensitivity to stress, inflammation, infections, and dietary change, and why psychiatric stability cannot be achieved unless zinc, copper, and ceruloplasmin dynamics are understood and corrected together.
TOXIC OVERLOAD / OXIDATIVE STRESS — Nutrition and Depression
In the Walsh Protocol, toxic overload is defined primarily by elevation of S-adenosylhomocysteine (SAH) and a reduced SAM:SAH ratio, rather than by simple deficiency of methyl donors. SAH is a potent competitive inhibitor of methyltransferase enzymes; when SAH accumulates, methylation reactions slow or stall even when SAM levels appear adequate. This biochemical pattern provides strong evidence of toxic inhibition of methylation, not merely insufficient nutrient supply.
Walsh emphasized that zinc is central to restoring methylation capacity in toxic overload, due to its role in supporting S-adenosylhomocysteine hydrolase (SAHH), the enzyme responsible for converting SAH into homocysteine and adenosine. When zinc is deficient or functionally impaired, SAHH activity declines, SAH accumulates, and methylation becomes progressively inhibited. This mechanism explains why toxic burden often presents with symptoms of depression, cognitive slowing, emotional blunting, and poor treatment response despite adequate methyl donors.
This pattern differs fundamentally from simple low SAM states. In toxic overload, relative SAH elevation suppresses methylation globally, impairing DNA and histone methylation, neurotransmitter metabolism, and histamine clearance. Walsh identified environmental toxins, heavy metals, chronic inflammation, and oxidative stress as common contributors that both raise SAH and increase the body’s demand for methylation while simultaneously blocking its execution.
Within this framework, Second Opinion Physician expands the Walsh model by addressing downstream clearance and metabolic bottlenecks that influence SAH and homocysteine handling once SAHH function is supported. SOP evaluates renal and hepatic disposal of homocysteine, recognizing that ineffective clearance can drive reconversion to SAH and perpetuate methylation inhibition. Alkalination strategies are used to support renal handling, while liver-directed support aids sulfur metabolism and homocysteine decomposition.
SOP also considers creatine synthesis as a major methyl drain in individuals with normal methionine but persistently low SAM alongside signs of high creatine demand. Because endogenous creatine production consumes large amounts of methyl groups, inadequate creatine availability can worsen SAM depletion and indirectly raise the impact of SAH inhibition. Targeted creatine support is therefore used selectively to preserve methylation capacity.
Mitochondrial function further modulates this biotype. In individuals with elevated homocysteine and low glutathione or cystathionine, SOP looks beyond B6 or NAC supplementation alone. Impaired mitochondrial capacity increases oxidative stress, diverts sulfur metabolites, and limits efficient conversion of methionine to SAM, reinforcing the toxic-overload pattern.
Conversely, some patients present with low methionine availability, commonly seen in vegans, individuals with low muscle mass, malabsorption, or dysbiosis. In these cases, insufficient substrate for SAM synthesis compounds the inhibitory effects of elevated SAH. Chronic medication use, processed foods, environmental chemical exposure, and heavy-metal burden further increase methylation demand while impairing enzymatic function.
In this context, nutrition and depression are inseparable from detoxification physiology and methylation balance. Walsh’s model centers on restoring zinc-dependent SAHH activity and lowering SAH to re-enable methylation. SOP’s approach supports this foundation by addressing clearance, mitochondrial efficiency, and methyl demand so that nutrient therapy can function effectively in patients burdened by toxic overload.
Additional Common Modifiers in the Walsh Protocol
In addition to the primary biotypes, Walsh identified several common physiological modifiers that do not define a biotype on their own but can significantly aggravate depression, anxiety, and behavioral symptoms. Glucose dyscontrol, particularly chronic low blood glucose, is frequently observed and can trigger irritability, anxiety, tremulousness, poor concentration, and post-meal drowsiness; treatment emphasizes dietary stabilization with frequent small meals rich in protein and complex carbohydrates, supported by targeted nutrients such as chromium and CoQ-10. Toxic substance exposure, including heavy metals and organic chemicals, may underlie symptoms in susceptible individuals—especially those with metallothionein dysfunction or overmethylation—and requires a three-part approach of exposure avoidance, biochemical support to enhance elimination, and correction of underlying nutrient imbalances to reduce future vulnerability. Malabsorption, involving impaired stomach acid, incomplete digestion, or brush-border dysfunction, can lead to nutrient deficiencies, inflammation, altered neurotransmission, and cognitive or behavioral impairment; treatment is individualized and may include digestive enzymes, probiotics, HCl adjustment, and specific dietary modifications. Finally, essential fatty acid imbalance is recognized as a critical factor affecting brain structure and neurotransmission, with omega-3 and omega-6 oils playing distinct and necessary roles; Walsh emphasized biochemical individuality in fatty acid needs, noting that supplementation should be guided by symptom patterns and specialized testing rather than applied uniformly.
FAQ's Nutrient Therapy in Depression - The Five Biotypes of Depression
Why Is the Walsh Protocol a Keystone for Nutrient Therapy in Depression and Anxiety?
The Walsh Protocol is a foundational framework for nutrient therapy for mood disorders because it links symptoms to measurable biochemical patterns. It explains why nutrients for depression and anxiety must be selected, avoided, or titrated based on biotype—and why generic supplementation often fails or worsens symptoms.
How Does Nutrition and Depression Differ by Biotype?
In the Walsh model, nutrition and depression are inseparable, but nutrition is never generic. The same nutrient may help one biotype and harm another. Effective treatment depends on understanding how diet and supplements interact with methylation, neurotransmitter transporters, mineral balance, and detoxification in each biotype.
What Is Undermethylation Depression and How Does It Affect Mood?
Undermethylation depression is characterized by low functional serotonin and dopamine activity, high histamine, and a low SAM:SAH ratio. These individuals often experience rumination, rigidity, seasonal depression, and inner tension. Certain nutrients—especially folates—can worsen symptoms by further reducing neurotransmitter availability.
What Is Overmethylation and Why Is It Linked to Anxiety and Panic?
Overmethylation depression and anxiety involve efficient or excessive methylation, low histamine, and heightened serotonergic activity. Panic, sensory sensitivity, insomnia, and emotional overstimulation are common. This biotype often responds differently to nutrients for depression and anxiety than undermethylation, highlighting the need for correct classification.
How Does Copper Overload Cause Anxiety and Emotional Instability?
Copper overload depression is driven by excess free copper relative to zinc and ceruloplasmin. Copper increases norepinephrine activity and weakens inhibitory control, producing anxiety, panic, agitation, and insomnia. Hormonal factors, genetics, and zinc deficiency commonly contribute to this biotype.
Why Is Pyrrole Disorder So Closely Linked to Copper and Zinc Imbalance?
Pyrrole disorder (pyroluria) causes chronic loss of zinc and vitamin B6, which destabilizes copper regulation and often leads to secondary copper overload. The clinical impact depends less on pyrroles alone and more on the resulting zinc, copper, and ceruloplasmin imbalance, making mineral assessment essential before treatment.
What Is Toxic Overload Depression and Why Do Supplements Sometimes Fail?
Toxic overload depression occurs when inflammation, metals, or environmental stress elevate SAH and suppress methylation efficiency. Even with adequate nutrients, methylation cannot function properly. This explains fatigue-dominant depression, brain fog, and poor response to supplements unless detoxification pathways are supported.
Why Can the Wrong Nutrients Worsen Depression or Anxiety?
In nutrient psychiatry, nutrients act like regulators, not vitamins. Folates, methyl donors, zinc, niacin, and amino acids can all worsen symptoms if used in the wrong biotype. The Walsh Protocol explains why supplements must be individualized to avoid increasing anxiety, depression, or emotional instability.
Why Is Individualized Nutrient Therapy Essential for Mental Health?
The Walsh Protocol shows that nutrient deficiency and mental health are governed by biochemical individuality. Identifying the correct biotype allows nutrients to be used precisely—to stabilize mood, reduce anxiety, and restore resilience—rather than applied generically with unpredictable or harmful effects.