Toxic Overload and Depression: The Effect of Toxins on Methylation and Glutathione Depletion
This article delves into the relationship between toxins, methylation pathways, and depression. Methylation is a crucial biochemical process for various bodily functions, including detoxification, gene expression, and neurotransmitter synthesis. However, toxins such as environmental pollutants, drugs, and chemicals in food can impair the methylation pathway by interfering with the enzymes involved in the process and depleting the body's stores of crucial components like SAMe and methionine. As a result, methylation may be disrupted, leading to a range of health issues, including mood disorders associated with undermethylation and copper overload. This article also discusses the impact of toxins on glutathione, a vital antioxidant that is involved in protecting cells from oxidative stress and detoxifying harmful substances like heavy metals, drugs, and environmental toxins. A decrease in glutathione levels can lead to a decrease in methylation, which can have serious implications for cellular health and function. A list of common foods and chemicals that can contribute to toxic overload is shown along with their common source and the detoxification pathway they engage.
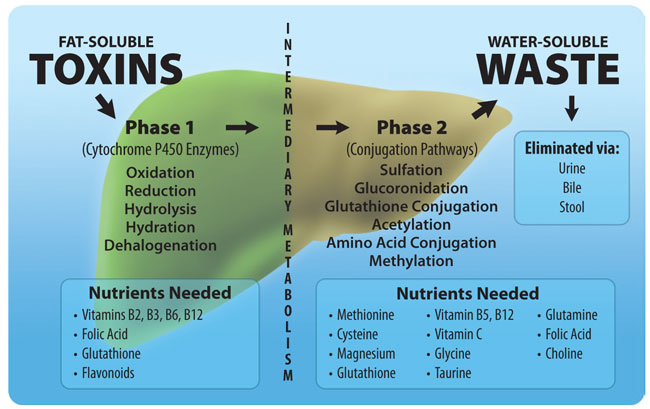
The Body's Primary Detoxification Pathways:
| Detox Mechanism Affected | Biochemical Processes Affected |
| Methylation | DNA Methylation, Histone Methylation, Neurotransmitter Synthesis, Myelin Production, Detoxification of Toxins and Drugs |
| Glutathione | Detoxification of Toxins and Drugs, Protection Against Oxidative Stress, Immune Function |
| P450 | Detoxification of Toxins and Drugs, Metabolism of Drugs, Steroid Hormones, and Cholesterol |
| Metallothionein | Detoxification of Heavy Metals, Regulation of Zinc and Copper Levels, Protection Against Oxidative Stress |
| Ceruloplasmin | Regulation of Iron Levels, Protection Against Oxidative Stress |
Methylation Pathway Disruption
Methylation is a vital biochemical process that occurs in every cell of the body, involving the transfer of a methyl group (CH3) from one molecule to another. It has various critical functions, including DNA synthesis, gene expression, neurotransmitter synthesis, and detoxification. Additionally, methylation plays a crucial role in epigenetic modifications, which can alter the expression of genes without changing the DNA sequence. This process is essential for maintaining overall health and wellbeing, regulating neurotransmitters, hormones, and enzymes, and controlling gene expression. Gene expression, influenced by methylation, is the primary mechanism of influence on the activity of serotonin and dopamine per the Walsh Approach, practiced here by Second Opinion Physician, to treating mood disorders.
Primary Benefits of Methylation
Methylation is involved in many critical bodily functions. Some of the primary benefits of methylation include:
- DNA synthesis and repair
- Gene expression
- Neurotransmitter synthesis
- Hormone regulation
- Detoxification
- Immune system function
- Energy production
Toxins and Their Impact on Methylation, Glutathione, and Antioxidant Production
Toxins, including environmental pollutants, drugs, and chemicals in food, can impair the methylation pathway by interfering with the enzymes involved in the process. Toxins can also deplete the body's stores of SAMe and methionine, which are crucial components of the methylation pathway. As a result, methylation may be disrupted.
SAMe and Methionine Depletion on the Body
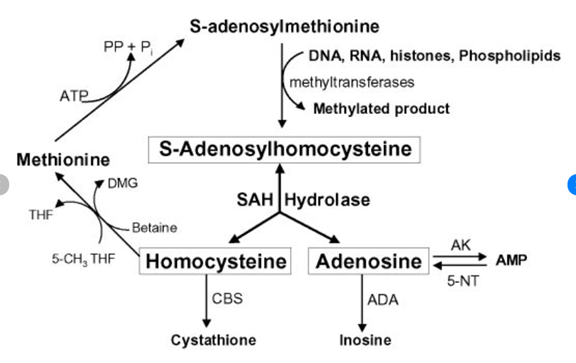
SAMe (S-adenosylmethionine) and methionine are essential components of the methylation pathway, a critical process involved in many essential bodily functions. SAMe is the primary methyl donor in the body, while methionine is an essential amino acid that is converted to SAMe in the body. Depletion of SAMe and methionine can have significant consequences for health, disrupting the methylation pathway and leading to various health issues.
Impact on DNA Synthesis, Repair, and Cancer Risk
Low levels of SAMe and methionine can impair DNA synthesis and repair, leading to genetic mutations and increasing the risk of cancer. The methylation of DNA plays a crucial role in regulating gene expression and maintaining genomic stability. Depletion of SAMe and methionine can lead to changes in DNA methylation patterns, potentially leading to the formation of cancer cells.
Impact on Neurotransmitter Synthesis and Mood Disorders
SAMe is involved in the synthesis of neurotransmitters, which are essential for maintaining a balanced mood and cognitive function. Depletion of SAMe and methionine can lead to imbalances in neurotransmitters, leading to mood disorders and cognitive dysfunction. Additionally, SAMe has been shown to have antidepressant effects and may be useful in treating depression and anxiety disorders.
Undermethylation: Genetic Predisposition or Environmental Depletion of Methyl Compounds?
The Liver's Detoxification Process: The Role of Methylation and Glutathione
The liver plays a vital role in the body's detoxification processes, and two important mechanisms it employs are methylation and glutathione. Methylation involves the addition of a methyl group to a harmful substance, which makes it more water-soluble and easier to excrete from the body. Enzymes called methyltransferases catalyze this process, using SAMe as a methyl donor. Meanwhile, glutathione uses a process called reduction to eliminate free radicals and harmful substances. It donates an electron to the harmful substance, neutralizing it and turning it into a less harmful compound. Glutathione also binds to harmful substances, such as heavy metals, drugs, and environmental toxins, making them easier to excrete from the body. These two mechanisms work together to effectively detoxify harmful substances in the liver.
The Relationship between Methylation and Glutathione
Methylation is necessary for the synthesis of glutathione. The production of glutathione relies on SAMe, which is produced through the methylation pathway. Methylation is also involved in the recycling of glutathione, which is required to eliminate free radicals and harmful substances from the body. Therefore, maintaining healthy levels of SAMe and glutathione is crucial for optimal liver function and effective detoxification.
Impact of Toxins on Methylation and Glutathione
Exposure to toxins, drugs, and chemicals in food can impact glutathione levels and methylation. Heavy metals, such as lead, mercury, and cadmium, can deplete glutathione levels, impairing the body's ability to detoxify harmful substances and reducing the availability of SAMe for methylation. Exposure to environmental toxins such as pesticides and air pollutants can also impact glutathione levels, leading to a decrease in methylation. Certain drugs, such as aspirin and ibuprofen, have been shown to deplete glutathione levels, impairing the body's ability to detoxify harmful substances and reducing the availability of SAMe for methylation. Similarly, alcohol consumption has been shown to reduce glutathione levels, leading to a decrease in methylation.
The Health Implications of Reduced Methylation and Glutathione Levels
The impact of toxins on methylation and glutathione can have serious implications for health. Reduced glutathione levels can lead to increased oxidative stress and impaired detoxification, while decreased methylation can lead to a range of health issues, including mood disorders, cognitive dysfunction, and cancer. Therefore, it is important to prioritize reducing exposure to toxins and supporting optimal detoxification mechanisms to maintain overall health and prevent chronic disease.
Other therapies and beyond antioxidants that can provide significant help to the body's detoxificaton process include ozone therapy, dry sauna treatments, exercise, hydrogen therapy, colon irrigation and liver cleansing.
The Role of P450 Enzymes in Detoxification and Methylation Pathways
In addition to the previously discussed detoxification pathways of methylation and glutathione, P450 enzymes also play a crucial role in detoxification. P450 enzymes are responsible for the oxidation of toxic compounds, making them more water-soluble and easier for the body to excrete. The liver is the primary site of P450 enzyme activity, and there are over 50 different P450 enzymes with unique substrate specificity.
P450 enzymes are involved in the metabolism of a wide range of compounds, including drugs, steroid hormones, fatty acids, and bile acids. These enzymes are responsible for the metabolism of both exogenous and endogenous compounds. P450 enzymes are also involved in the first step of the methylation pathway, where they convert methionine to S-adenosylmethionine (SAMe).
The Role of P450 in Methylation
SAMe is a critical molecule in the body, playing a key role in the methylation of DNA, RNA, proteins, and neurotransmitters. P450 enzymes are involved in the conversion of methionine to SAMe, which is a critical component of the methylation pathway. The methylation of DNA is particularly important, as it helps to regulate gene expression and maintain the integrity of the genome.
Impact of Toxins on P450 Enzymes and Methylation
Exposure to toxins, drugs, and alcohol can impair the activity of P450 enzymes, leading to a decrease in SAMe production and impaired methylation. Polycyclic aromatic hydrocarbons (PAHs) found in cigarette smoke, for example, have been shown to decrease the activity of P450 enzymes, leading to a decrease in SAMe production and impaired DNA methylation.
Similarly, some drugs can impact P450 enzyme activity. Rifampin, an antibiotic, has been shown to increase the activity of P450 enzymes, leading to an increase in SAMe production and increased DNA methylation. Conversely, the antifungal drug ketoconazole has been shown to inhibit P450 activity, leading to a decrease in SAMe production and impaired methylation.
Reduced P450 enzyme activity can lead to a decrease in SAMe production, which can impair the methylation pathway. Impaired methylation can have significant consequences for health, including mood disorders, cognitive dysfunction, and cancer.
Copper Overload, Metallothionein, Ceruloplasmin, and Zinc Deficiencies: The Effects on Methylation and Glutathione Pathways
Copper is a vital mineral in the body, playing a crucial role in various biological processes. However, excessive levels of copper in the body can lead to a condition called copper overload, which can have severe consequences. Copper overload can affect the methylation pathway, which can have a domino effect on various other systems in the body.
Metallothionein and Ceruloplasmin
Metallothionein is a protein that binds to metals such as copper and zinc, playing a crucial role in their regulation in the body. It also acts as an antioxidant and scavenges free radicals. On the other hand, ceruloplasmin is a protein that carries copper in the bloodstream. The balance between copper and zinc is essential for the proper functioning of metallothionein and ceruloplasmin. If this balance is disrupted, it can lead to copper overload and zinc deficiency.
Effects of Copper Overload on Methylation and Glutathione Pathways
Copper overload can have a significant impact on the methylation pathway. High levels of copper can lead to increased levels of oxidants, causing damage to DNA and other cellular components. Elevated copper levels can also lead to a decrease in S-adenosylmethionine (SAMe) and glutathione levels, which can affect methylation and toxin removal from the body. This can lead to further accumulation of toxins, which can cause further damage to DNA and other cellular components.
Furthermore, copper can stimulate the activity of an enzyme called cystathionine beta-synthase (CBS), which converts homocysteine to cystathionine in the methionine cycle. This can lead to an increase in homocysteine levels, which can inhibit the activity of methionine synthase (MS), the enzyme that converts homocysteine to methionine in the methylation cycle. As a result, SAMe production is decreased, which, in turn, can lead to decreased glutathione synthesis since SAMe is required for the production of glutathione.
Moreover, excess copper can directly oxidize glutathione, leading to its depletion. When copper is in excess, it can cause oxidative stress and damage to cells, which increases the demand for glutathione to neutralize the free radicals produced. This can lead to the depletion of glutathione, which can further compromise the body's ability to detoxify toxins and heavy metals.
Effects of Zinc Deficiency on Methylation and Glutathione Pathways
Zinc deficiency can lead to a decrease in ceruloplasmin activity, leading to a decrease in copper levels. This can also have a significant impact on the methylation pathway. Zinc deficiency can lead to a decrease in SAMe levels, which can lead to impaired methylation. Zinc is also involved in the production of metallothionein, and a deficiency in zinc can lead to decreased metallothionein production, further exacerbating copper overload.
Effects of Copper and Zinc Imbalances on Neurotransmitters
The imbalances in copper and zinc can also have an impact on neurotransmitter levels. Dopamine and norepinephrine are catecholamines that are involved in the regulation of mood and behavior. Zinc deficiencies, which are very common, lead to increased copper levels, as well as low ceruloplasmin, all of which translates into elevated 'free copper'. Free copper is a cofactor in the conversion of dopamine to noradrenalin in the brain. Such an imbalance is a primary driver for mood disorders such as anxiety, insomnia, paranoia, irritability, depression and psychosis, as well as common conditions such as ADHD, Panic Disorders and Post Partum Depression.
Foods, Chemicals Sources in Our Diet and Environment plus Detoxification Pathway Required to Detoxify:
| Chemical | Sources | Common Foods/Drugs | Detox System Affected |
| Acetaminophen | Pain reliever | Over-the-counter pain relievers | Glutathione |
| Alcohol | Beverage | Beer, wine, spirits | Methylation & Glutathione |
| Antibiotics | Medication | Prescribed antibiotics, antibiotic residues in food | Methylation |
| Antidepressants | Medication | SSRIs, MAOIs, tricyclic antidepressants | Methylation, P450 |
| Antipsychotics | Medication | Typical and atypical antipsychotics | Methylation, P450 |
| Artificial food dyes | Food coloring | Candy, soda, processed foods | Methylation & Glutathione |
| Aspartame | Artificial sweetener | Diet soda, sugar-free gum, other artificially sweetened products | Methylation & Glutathione |
| Bisphenol A (BPA) | Plastics, canned foods, cash register receipts | Canned foods, plastic containers, thermal paper receipts | Methylation & Glutathione |
| Caffeine | Stimulant | Coffee, tea, energy drinks | Methylation |
| Glyphosate | Herbicides | GMO crops, conventionally grown oats and wheat, wine, beer Also found in some non-GMO crops that are desiccated with glyphosate before harvesting. | Methylation & Glutathione |
| Heavy metals (lead, mercury, cadmium) | Pollution, contaminated water and soil | Shellfish, contaminated fish, cigarette smoke | Glutathione, P450, Metallothionein |
| Melatonin | Hormone | Produced naturally in the body, also available as a dietary supplement | Methylation & Glutathione |
| Monosodium glutamate (MSG) | Flavor enhancer | Found in a wide variety of processed foods, including chips, soups, snack foods, and Chinese food. Also found in some protein powders and other dietary supplements. | Methylation & Glutathione |
| Nicotine | Stimulant | Cigarettes, vaping products | Methylation |
| NSAIDs (nonsteroidal anti-inflammatory drugs) | Medication | Over-the-counter pain relievers | Methylation & Glutathione |
| P450 | Enzymes | Involved in metabolizing many drugs and toxins | P450 |
| Perfluorooctanoic acid (PFOA) | Nonstick cookware, stain-resistant fabrics | Fast food wrappers, nonstick cookware, microwave popcorn bags | Methylation & Glutathione |
| Pesticides | Insecticides, herbicides, fungicides | Conventionally grown produce, non-organic grains | Methylation & Glutathione |
| Phthalates | Plastics, personal care products | Vinyl flooring, plastic food containers, personal care products | Methylation & Glutathione |
| Polychlorinated biphenyls (PCBs) | Industrial chemicals, electrical equipment | Farmed salmon, contaminated fish, electrical equipment | Methylation & Glutathione |
| Polycyclic aromatic hydrocarbons (PAHs) | Exhaust fumes, cigarette smoke, charred food | Grilled or charred food, cigarette smoke | Methylation & Glutathione |
| SAMe | Dietary supplement | Supplements and some foods, such as broccoli and spinach | Methylation |
| Statins | Medication | Cholesterol-lowering drugs | Methylation |
| Stimulants | Medication | ADHD medications, cocaine, methamphetamine | Methylation, P450 |
| Sulfites | Preservative | Wine, dried fruit, processed foods | Methylation |
| Volatile organic compounds (VOCs) | Cleaning products, paints, solvents | Cleaning products, paints, solvents | Methylation & Glutathione |
The Importance of Gut Microbiome in Detoxification Pathways and Obesity
The gut microbiome has a crucial role in maintaining our overall health, including supporting optimal detoxification mechanisms. Intestinal dysbiosis, which refers to an imbalance in the gut microbiome, has been linked to several health conditions, including obesity. Studies suggest that dysbiosis may affect the metabolism of dietary components, increase inflammation, and alter gut hormone signaling, leading to the development of obesity. Additionally, obesity itself can contribute to gut dysbiosis, creating a vicious cycle.
Endotoxins and Detoxification Mechanisms
Endotoxins are produced by certain bacteria in the gut, especially gram-negative bacteria, and can enter the bloodstream when the gut lining is compromised. This can lead to systemic inflammation and a range of symptoms, including fatigue, brain fog, headaches, joint pain, bloating, gas, and diarrhea. Endotoxin exposure can also contribute to the development of chronic diseases such as metabolic syndrome, diabetes, and cardiovascular disease.
Inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and leaky gut syndrome are some of the intestinal pathologies that can be caused by endotoxin exposure. In these conditions, the gut lining is compromised, allowing endotoxins and other antigens to leak into the bloodstream and trigger an immune response. The impact of endotoxins and leaking antigens on detoxification mechanisms can be significant. They can increase oxidative stress and inflammation, disrupt the gut microbiome, and impact the methylation pathway. These effects can lead to damage to cells and tissues, further burdening the liver and other organs involved in detoxification.
Effects of Endotoxins on Methylation Pathway and Glutathione Production
Endotoxin exposure can increase the production of pro-inflammatory cytokines, such as TNF-α, which can lead to an upregulation of CBS enzyme. This enzyme converts homocysteine to cystathionine in the methionine cycle, which can increase homocysteine levels. Elevated homocysteine levels can inhibit the activity of MS enzyme, leading to decreased SAMe production. SAMe is essential for the production of glutathione, which is crucial for detoxification. Endotoxins can also cause oxidative stress, increasing the demand for glutathione to neutralize free radicals produced. This can lead to the depletion of glutathione, further compromising the body's ability to detoxify toxins and heavy metals.
Promoting a Healthy Gut Microbiome for Optimal Detoxification
Addressing gut dysbiosis and reducing the production of endotoxins and leaking antigens is crucial for maintaining optimal health and supporting the body's detoxification mechanisms. It is essential to promote a healthy gut microbiome by consuming a balanced diet with fiber-rich foods, avoiding processed foods, and taking probiotics or fermented foods. Regular exercise and stress management techniques can also support a healthy gut microbiome.
Other Factors Affecting Methylation and Metal Management in the Body
Gluten and Casein Allergies
Food can also affect methylation and metal management by the body. Gluten is a protein found in wheat, barley, and rye, and casein is a protein found in milk and dairy products. Some individuals have an immune response to these proteins, leading to inflammation and gut dysbiosis. This inflammation can impact the methylation cycle, leading to decreased production of SAMe and glutathione. In addition, gut dysbiosis can also lead to increased levels of heavy metals and other toxins in the body, further compromising the body's ability to detoxify. However, while gluten and casein allergies can have an impact on detoxification, it is important to note that not everyone with these allergies will have methylation or metal management issues. It is also important to work with a healthcare provider to properly diagnose and address any food allergies or sensitivities.
Nutritional Deficiencies or Excesses
Diet and intestinal function can also affect methylation and metal management. For example, deficiencies in vitamins B6, B12, and folate can impair methylation processes, as these vitamins are required for the production of SAMe, a key methyl donor. On the other hand, excessive intake of certain nutrients, such as iron and copper, can increase oxidative stress and disrupt metal homeostasis, leading to tissue damage and impaired detoxification. It is important to maintain a balanced and varied diet to ensure adequate intake of all essential nutrients, and to avoid excessive intake of potentially harmful substances.
Stress and Adrenal Function
Chronic stress can have a variety of negative effects on the body, including the potential to increase cortisol levels. Cortisol is a hormone that is released by the adrenal gland in response to stress, and it plays a role in a number of physiological processes, including glucose metabolism and immune system function. Cortisol also has an impact on methylation and detoxification processes. Both excess and deficient levels of cortisol can disrupt the methylation cycle and affect the body's ability to detoxify harmful substances.
Excess cortisol levels, also known as hypercortisolism or Cushing's syndrome, can lead to a range of health issues, including impairments in methylation and detoxification. High cortisol levels can lead to depletion of glutathione, a critical antioxidant involved in the detoxification of harmful substances. Glutathione depletion can lead to increased oxidative stress, DNA damage, and impaired detoxification. Additionally, high cortisol levels have been shown to reduce SAMe levels, which are crucial components of the methylation pathway.
On the other hand, deficient cortisol levels, also known as hypocortisolism or Addison's disease, can also affect methylation and detoxification. Low cortisol levels can lead to an increase in inflammation, which can negatively impact methylation and impair detoxification mechanisms. Low cortisol levels have also been associated with an increase in oxidative stress and a decrease in glutathione levels, further impairing detoxification.
In Summary
In conclusion, this article has shed light on the harmful effects of toxins on metabolic pathways, leading to undermethylation and copper overload, which can ultimately lead to mood disorders and depression. The Walsh Approach to Depression recognizes Toxic Overload as a significant contributor to depression and emphasizes the importance of minimizing exposure to chemicals and maintaining a healthy diet. It is crucial to prioritize gut health and reduce exposure to toxins to support optimal detoxification and overall health. If you are looking for ways to mitigate the impact of toxins on your health, check out our follow-up article on diet and lifestyle therapies that can help.